How Can The Enthalpy Change Be Determined For A Reaction In An Aqueous Solution
Enthalpy of Solution
- Page ID
- 1623
A solution is a homogeneous mixture of two or more substances and tin can either be in the gas stage, the liquid phase, the solid phase. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at constant pressure level). This enthalpy of solution (\(ΔH_{solution}\)) can either be positive (endothermic) or negative (exothermic). When understanding the enthalpy of solution, it is easiest to remember of a hypothetical three-step process happening between two substances. One substance is the solute, let'south call that A. The other substance is the solvent, let's call that B.
Step i: Breaking up the Solute
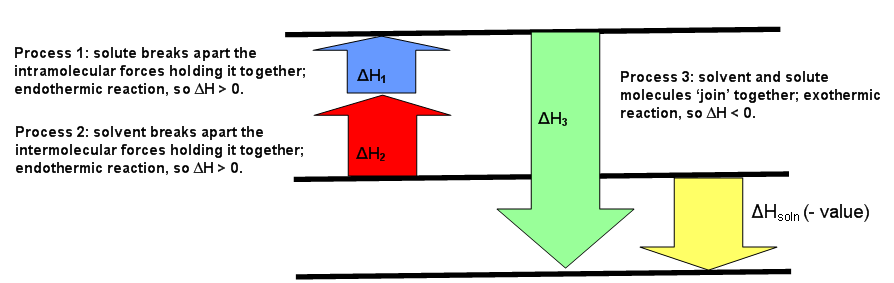
The starting time procedure that happens deals but with the solute, A, which requires breaking all intramolecular forces belongings it together. This means the solute molecules split from each other. The enthalpy of this procedure is chosen \(ΔH_1\). This since this is e'er an endothermic process (requiring energy to pause interactions), then \(ΔH_1 > 0\).
\[ \ce{A (southward) ->[\text{free energy in}] A (g)} \nonumber \]
Step 2: Breaking upward the Solvent
The 2d process is very similar to the showtime step. Much like how the solute, A, needed to suspension apart from itself, the solvent, B, besides needs to overcome the intermolecular forces holding it together. This causes the solvent molecules separate from each other. The enthalpy of this process is called \(ΔH_2\). Similar the showtime step, this reaction is always endothermic (\(ΔH_2 > 0\)) because energy is required to intermission the interaction between the B molecules.
\[ \ce{B (l) ->[\text{energy in}] B (g)} \nonumber \]
At this point, let us visualize what has happened then far. The solute, A, has broken from the intermolecular forces holding it together and the solvent, B, has cleaved from the intermolecular forces holding it together too. Information technology is at this time that the third process happens. We also have ii values \(ΔH_1\) and \(ΔH_2\). that are both greater than cypher (endothermic).
Step iii: Combining the Ii Together
The third process is when substance A and substance B mix to for a solution. The separated solute molecules and the separated solvent molecules join together to grade a solution. This solution volition contain one mole of the solute A in an infinite amount of the solvent B.The enthalpy of combining these 2 substances to form the solution is \(ΔH_3\) and is an exothermic reaction (releasing heat since interactions are formed) with \(ΔH_3 < 0\).
\[ \ce{A (1000) + B (g) ->[\text{energy out}] A(sol)} \nonumber \]
The enthalpy of solution can expressed as the sum of enthalpy changes for each step:
\[ΔH_{solution} = ΔH_1 + ΔH_2 + ΔH_3. \label{eq1}\]
Then the enthalpy of solution tin can either be endothermic, exothermic or neither \(ΔH_{solution} = 0\)), depending on how much oestrus is required or release in each step. If \(ΔH_{solution} = 0\), then the solution is chosen an platonic solution and if \(ΔH_{solution} > 0\) or \(ΔH_{solution} < 0\), and then these solutions are chosen non-platonic solutions.
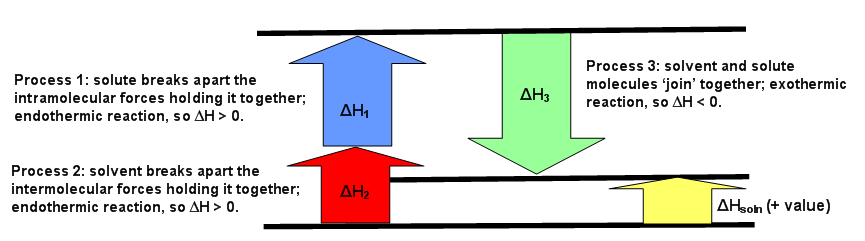
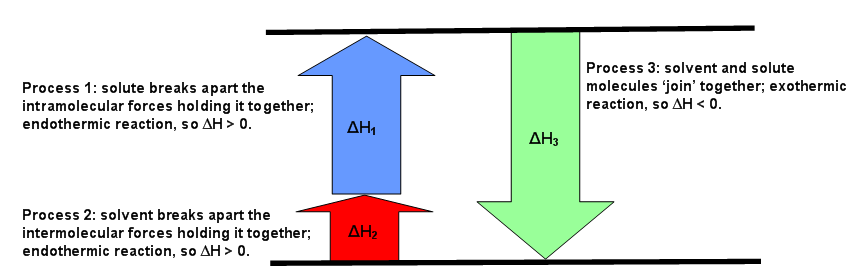
The diagrams below can be used every bit visuals to aid facilitate the agreement of this concept. Effigy \(\PageIndex{1}\) is for an endothermic reaction, where \(ΔH_{solution} > 0.\) Figure \(\PageIndex{two}\) is for an exothermic reaction, where \(ΔH_{solution} < 0\). Effigy \(\PageIndex{3}\) is for an ideal solution, where \(ΔH_{solution} = 0\).
Platonic Solutions
The enthalpy of solution depends on the strengths of intermolecular forces of the solute and solvent and solvent (Equation \ref{eq1}). If the solution is platonic, and \(ΔH_{solution} = 0\), then
\[\brainstorm{marshal*} ΔH_{solution} = ΔH_1 + ΔH_2 + ΔH_3 &= 0. \label{eq2} \\[4pt] ΔH_1 + ΔH_2 &= - ΔH_3 \cease{align*}\]
This means the forces of attraction between like (the solute-solute and the solvent-solvent) and unlike (solute-solvent) molecules are the same (Figure \(\PageIndex{three}\)). If the solution is not-platonic, so either \(ΔH_1\) added to \(ΔH_2\) is greater than \(ΔH_3\) or \(ΔH_3\) is greater than the sum of \(ΔH_1\) and \(ΔH_2\). The first instance means the forces of attraction of unlike molecules is greater than the forces of allure between similar molecules. The second case means the forces of attraction between similar molecules is greater than the forces of attraction between different molecules (Figure \(\PageIndex{2}\)).
Example \(\PageIndex{1}\): Table table salt
Table salt (\(\ce{NaCl}\)) dissolves readily in water. In solid (\(\ce{NaCl}\)), the positive sodium ions are attracted to the negative chloride ions. The same is true of the solvent, water; the partially positive hydrogen atoms are attracted to the partially negative oxygen atoms. While (\(\ce{NaCl}\)) dissolves in water, the positive sodium cations and chloride anions are stabilized past the water molecule electrical dipoles. Thus, the intermolecular interactions (i.east., ionic bonds) betwixt (\(\ce{NaCl}\)) are broken and the salt is dissolved. The overall chemical equation for this reaction is as follows:
\[\ce{NaCl (south) ->[H_2O] Na^+ (aq) + Cl^- (aq)}\]
Enthalpy of solution is only one part of the driving force in the germination of solutions; the other role is the entropy of solution.
References
- Petrucci, Harwood, Herring, Madura. General Chemical science: Principles & Modern Applications, Ninth Ed. Upper Saddle River, NJ: Pearson Education, Inc., 2007.
- McMurray, Fay. Chemistry, Third Ed. Upper Sadle River, NJ: Prentice-Hall, Inc., 2001.
Contributors and Attributions
- Zafir Javeed, Mark Tye (DVC)
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Solutions_and_Mixtures/Solution_Basics/Enthalpy_of_Solution
Posted by: thorntonxvier1937.blogspot.com

0 Response to "How Can The Enthalpy Change Be Determined For A Reaction In An Aqueous Solution"
Post a Comment